Table of Contents
- What Materials Are Used for Medical Yarns? Introduction: The Critical Role of Material Science in Modern Medical Textiles
- 1.1 From Surgical Sutures to Implants: Defining the Scope of Medical Yarns
- 1.2 The Uncompromising Requirements: Biocompatibility, Sterilizability, and Performance
- The Foundation: Core Principles of Medical Material Selection
- 2.1 Biocompatibility & Toxicology: ISO 10993 and USP Class VI Standards
- 2.2 The Absorption Profile: Biodegradable vs. Non-Biodegradable Materials
- 2.3 Mechanical Property Requirements: Strength, Elasticity, and Knot Security
- Material Deep Dive: A Taxonomy of Medical Yarn Polymers
- 3.1 Synthetic Absorbable Polymers: PGA, PLGA, Polydioxanone (PDS)
- 3.2 Synthetic Non-Absorbable Polymers: Polypropylene, Polyester, PTFE, UHMWPE
- 3.3 Natural and Regenerated Polymers: Silk, Cotton, Collagen, Chitosan
- 3.4 Specialty & Engineered Materials: Silver-coated, Drug-eluting, Shape Memory Alloys
- Comparative Analysis: Performance Data of Key Medical Yarn Materials
- 4.1 Table 1: Mechanical and Degradation Properties of Absorbable Sutures
- 4.2 Table 2: Characteristics and Applications of Non-Absorbable Sutures
- 4.3 Table 3: Key Properties and Uses of Advanced Medical Textile Yarns
- From Fiber to Function: Manufacturing and Processing Considerations
- 5.1 Spinning Techniques: Melt, Wet, and Dry Spinning for Medical Polymers
- 5.2 Yarn Construction: Monofilament, Multifilament, Braided, and Barbed
- 5.3 Coatings and Finishes: Enhancing Lubricity, Bioactivity, and Handling
- Application-Based Material Selection Guide
- 6.1 Surgical Sutures & Ligatures: Precision and Healing
- 6.2 Implantable Meshes: Hernia Repair, Pelvic Floor, Tendon Scaffolds
- 6.3 Advanced Wound Care: Bioactive Dressings and Tissue Engineering
- 6.4 Minimally Invasive Devices: Catheters, Stents, and Delivery Systems
- Regulatory Landscape and Quality Assurance
- 7.1 Navigating FDA (US), CE Marking (EU), and PMDA (Japan) Pathways
- 7.2 Essential Standards: ISO, ASTM, and Pharmacopoeia Monographs
- 7.3 Supply Chain Integrity: From Polymer Pellets to Sterile Packaging
- Innovations and Future Trends in Medical Yarn Materials
- 8.1 Bioresorbable Metals and Composites
- 8.2 Smart Yarns with Sensing and Actuation Capabilities
- 8.3 The Rise of Patient-Specific, 3D-Printed Implant Structures
- Strategic Sourcing and Supplier Evaluation for Industry Professionals
- 9.1 Critical Questions to Ask Medical Yarn Material Suppliers
- 9.2 Cost vs. Value Analysis in a Regulated Environment
- 9.3 Risk Management and Building a Compliant Supply Chain
- Conclusion: Making Informed Material Choices for Life-Saving Applications
- Frequently Asked Questions (FAQ)
1. Introduction: The Critical Role of Material Science in Modern Medical Textiles
For procurement managers and product developers in the broader textile industry, understanding the specialized world of medical yarns is no longer just a niche interest—it represents the frontier of high-value, technology-driven textile applications. The question “What materials are used?” lies at the heart of a multi-billion dollar global market where material choice is a direct determinant of clinical success, patient safety, and commercial viability. Unlike conventional textiles where aesthetics and durability reign, medical yarns operate under a paradigm of absolute biocompatibility, predictable performance in the harsh biological environment, and stringent regulatory oversight.
This guide is designed to bridge the knowledge gap for textile professionals looking to understand or potentially enter the medical field. We will move beyond simple material lists to explore the why and how behind material selection, providing a rigorous, data-driven framework that reflects the depth and complexity of this sector. From sutures that hold a heart together to meshes that rebuild abdominal walls, the materials profiled here are engineering marvels with life-altering impact.
2. The Foundation: Core Principles of Medical Material Selection
Selecting a material for a medical yarn is governed by non-negotiable principles that override cost and processability.
- Biocompatibility: The paramount requirement. A material must not elicit an adverse local or systemic reaction, cause toxicity, or be carcinogenic. This is rigorously evaluated per ISO 10993 (Biological Evaluation of Medical Devices) series, which includes tests for cytotoxicity, sensitization, and implantation.
- Sterilization Stability: The material must withstand sterilization (e.g., autoclaving, gamma irradiation, ethylene oxide) without degrading or losing its mechanical properties.
- Absorption Profile: Materials are categorized as:
- Biodegradable (Absorbable): Designed to degrade and be absorbed by the body over a predictable timeframe (weeks to months) as tissue heals. Ideal for internal sutures where removal is impractical.
- Non-Absorbable: Remain in the body permanently or require removal. Chosen for long-term support, as in hernia meshes or cardiovascular sutures.
- Mechanical Performance: Properties like tensile strength, elongation at break, knot pull strength, and creep resistance must be precisely matched to the physiological loads and movements of the target tissue.
3. Material Deep Dive: A Taxonomy of Medical Yarn Polymers
3.1 Synthetic Absorbable Polymers
These are the workhorses of modern internal surgery, engineered to hydrolyze into biocompatible metabolites.
- Polyglycolic Acid (PGA): A rigid, high-strength polymer that loses 50% of its strength in 2-3 weeks and is fully absorbed in 4-6 months. Known for its consistent, predictable degradation. Common Use: General soft tissue approximation.
- Poly(lactic-co-glycolic acid) (PLGA): A copolymer of lactic and glycolic acids. By varying the ratio, engineers can precisely tune the degradation rate from weeks to over a year. More flexible than pure PGA.
- Polydioxanone (PDS): A polyester with excellent flexibility and a longer absorption profile (maintains strength for ~6 weeks, fully absorbed in 6+ months). Common Use: Pediatric cardiothoracic surgery, where slow tissue growth is expected.
3.2 Synthetic Non-Absorbable Polymers
These provide permanent structural support and are chosen for their inertness and durability.
- Polypropylene (PP): A monofilament fiber with high chemical inertness, memory (resists creasing), and excellent tensile strength. It elicits a minimal fibrous tissue reaction. Common Use: The gold standard for hernia repair meshes and prolapse surgeries.
- Polyester (PET, e.g., Dacron®): A braided multifilament yarn that is exceptionally strong and promotes robust tissue ingrowth. Often coated with silicone or collagen to improve handling and reduce capillarity.
- Polytetrafluoroethylene (PTFE, e.g., GORE-TEX®): Extremely hydrophobic and biocompatible, with a low friction coefficient. Used in cardiovascular patches and sutures where blood compatibility is critical.
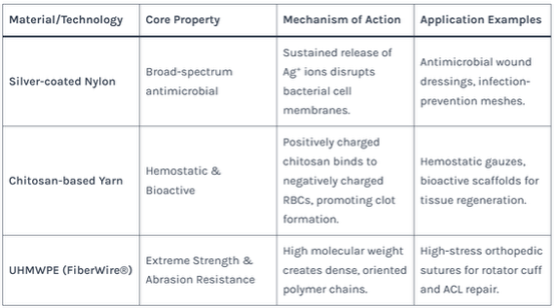
- Ultra-High-Molecular-Weight Polyethylene (UHMWPE): Possesses extraordinary strength and wear resistance. Used in high-performance orthopedic sutures for tendon and ligament repair (e.g., FiberWire®).
3.3 Natural and Regenerated Polymers
Leveraging nature’s own building blocks for specific applications.
- Silk: A natural protein fiber (from the silkworm Bombyx mori) with superb handling, knot security, and a moderate inflammatory response. It is slowly proteolyzed in the body over years. Common Use: Ophthalmic and neurological surgeries.
- Collagen: Derived from bovine or porcine tendons. It is highly biocompatible and can be processed into absorbable sutures or scaffolds that directly support cell migration.
- Chitosan: Derived from crustacean shells, this polysaccharide has inherent hemostatic (blood-clotting) and antimicrobial properties. Used in advanced wound dressings and hemostatic yarns.
3.4 Specialty & Engineered Materials
- Silver-coated Yarns: Silver ions are potent antimicrobials. Nylon or polyester yarns coated with silver are woven into dressings for managing infected or high-risk wounds.
- Drug-Eluting Yarns: Polymer yarns (e.g., PLGA) are loaded with active pharmaceuticals (antibiotics, anti-proliferatives) that release at the implant site over time to prevent infection or scar tissue formation.
- Shape Memory Alloys (Nitinol): While not a polymer, nickel-titanium wires are crucial “yarns” in self-expanding stents and orthopedic devices, offering superelasticity and the ability to return to a pre-set shape at body temperature.
4. Comparative Analysis: Performance Data of Key Medical Yarn Materials
Table 1: Mechanical and Degradation Properties of Common Absorbable Sutures
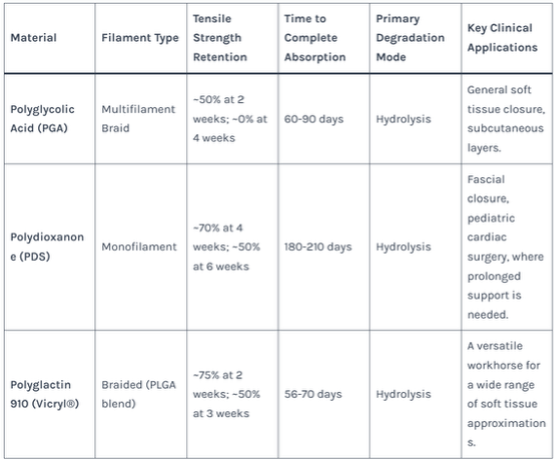
Table 2: Characteristics and Applications of Key Non-Absorbable Sutures
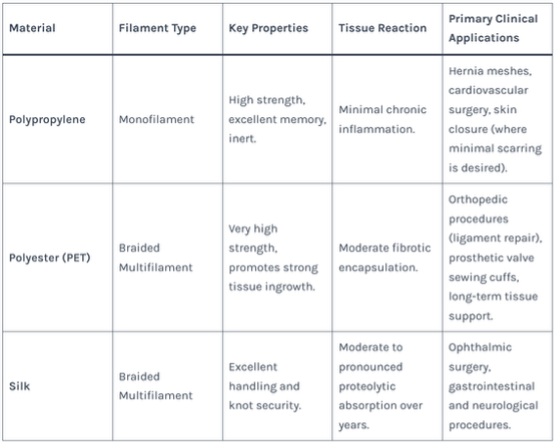
Table 3: Key Properties and Uses of Advanced Medical Textile Yarns
5. From Fiber to Function: Manufacturing and Processing Considerations
The journey from medical-grade polymer to functional yarn is a precision engineering challenge.
- Spinning: Most synthetic polymers are melt-spun (extruded through a spinneret). Absorbable polymers like PLGA require strict control of temperature and atmospheric moisture to prevent premature degradation. Wet spinning is used for some absorbables and regenerated polymers like chitosan.
- Yarn Construction: The choice is critical:
- Monofilament: Single strand. Smooth, resists bacterial adhesion, but can be stiff and prone to kinking (e.g., PDS, PP).
- Multifilament/Braided: Multiple filaments twisted or braided. Softer, more flexible, and has better knot security, but can wick fluids and bacteria if uncoated (e.g., Polyester, Silk).
- Coatings: Braided sutures are often coated to reduce tissue drag (“sawing”) and capillarity. Common coatings include silicone, wax, and absorbable polymers like polycaprolactone.
6. Application-Based Material Selection Guide
- Sutures:
- General Closure: PLGA (Vicryl) – balanced strength, handling, absorption.
- Fascial/Facial Closure: PDS or Polypropylene – for long-term tension support with minimal scarring.
- Microsurgery: Nylon or Polypropylene monofilament (10-0, 11-0 gauge) – for extreme fineness and low reactivity.
- Implantable Meshes:
- Hernia Repair (Standard): Lightweight, large-pore Polypropylene – reduces foreign body mass and allows for better tissue integration.
- Hernia Repair (Complex/Contaminated): Biological Mesh (e.g., cross-linked porcine dermis) or Absorbable Synthetic (e.g., Vicryl Mesh) – to reduce infection risk in compromised fields.
- Advanced Wound Care:
- Moist Management: Alginate yarns (from seaweed) form a gel upon contact with exudate.
- Anti-Biofilm: PHMB-coated polyester yarns provide persistent antimicrobial action.
7. Regulatory Landscape and Quality Assurance
Medical yarns are Class II or III medical devices. Bringing them to market requires:
- Regulatory Pathway: A 510(k) clearance (US FDA) or Technical File (EU MDR) demonstrating substantial equivalence to a predicate device and compliance with essential safety and performance requirements.
- Quality Systems: Suppliers must operate under a certified Quality Management System (e.g., ISO 13485), which governs every step from raw material receipt to sterile packaging.
- Traceability: Full traceability from the polymer resin lot to the final product batch is mandatory for recalls and post-market surveillance.
8. Innovations and Future Trends
- Bioresorbable Metals: Magnesium and iron alloy wires that provide strong temporary support before safely corroding in the body.
- Electroactive Yarns: Conductive polymers or coatings that can deliver electrical stimulation to promote wound healing or nerve regeneration.
- 3D Printing/Biofabrication: Using extruded “bio-inks” (often hydrogel-based yarns containing living cells) to print patient-specific tissue scaffolds directly.
9. Strategic Sourcing and Supplier Evaluation
Critical Questions for Suppliers:
- “Can you provide the Device Master File or CE Technical Documentation relevant to this yarn?”
- “What is your polymer sourcing strategy, and can you provide certificates of analysis for each resin lot?”
- “What sterilization method is validated for this product, and what is the resultant sterility assurance level (SAL)?”
- “What mechanical test data (ASTM F382, etc.) can you provide for this yarn lot?”
10. Conclusion
The material universe of medical yarns is a testament to the convergence of polymer science, biology, and clinical need. For textile professionals, understanding this landscape is not just an academic exercise; it is the key to participating in one of the most innovative and demanding sectors of the global textile industry. Success hinges on respecting the primacy of biocompatibility, navigating the rigorous regulatory environment, and partnering with suppliers who view quality as a non-negotiable predicate to function. The correct material choice, as detailed here, is ultimately what enables a simple strand of yarn to become a vital tool in healing and saving lives.
11. Frequently Asked Questions (FAQ)
Q1: Is surgical cotton the same as regular cotton?
A: No. Medical-grade “cotton” (often called Purified Cotton USP) undergoes rigorous chemical purification to remove natural waxes, pectins, and pigments. It is then processed, packaged, and sterilized in a controlled environment to ensure it is pyrogen-free and meets stringent standards for absorbency and fiber length, making it safe for wound contact.
Q2: Why are some sutures braided and others monofilament?
A: It’s a trade-off between handling and biocompatibility. Braided sutures are easier to handle, tie securely, and have better knot security but can potentially harbor bacteria in the interstices. Monofilament sutures are smoother, cause less tissue drag, and resist bacterial adhesion but are more difficult to handle and can retain memory (kinks).
Q3: What does “absorbable” really mean? Does the suture just disappear?
A: “Absorbable” means the suture undergoes controlled degradation (usually hydrolysis) into metabolic byproducts (like lactic acid) that the body can safely eliminate or incorporate. It doesn’t “vanish” but rather loses its mechanical integrity predictably and is metabolized over time, so removal is not required.
Q4: Can medical yarns be recycled or are they single-use only?
A: Virtually all implantable medical yarns are single-use, terminally sterilized devices due to the absolute requirement for sterility and performance integrity. Sustainability efforts focus on reducing packaging waste, using cleaner manufacturing processes, and, in rare cases, reprocessing certain external devices (like compression garments) under strict protocols.
Q5: What is the difference between a medical yarn and a regular textile yarn, chemically speaking?
**A: Chemically, they may be identical (e.g., both can be polyethylene terephthalate). The difference lies in the **purity, consistency, and documentation. Medical-grade polymers have tightly controlled molecular weight distributions, minimal impurities (e.g., catalysts, heavy metals), and are produced under current Good Manufacturing Practices with complete traceability—factors not typically controlled for general textiles.
Q6: How important is the color of a medical yarn?
A: Very important, but not for aesthetics. Suture colors (violet for Vicryl, blue for PDS, etc.) provide crucial visual contrast against tissue for the surgeon. Dyes used must be non-toxic and approved for medical use. For implants like meshes, colorants are often avoided to minimize any potential inert additive.
Q7: Are there latex-free medical yarn options?
A: Yes, absolutely. Due to high rates of latex allergy, modern medical yarns, especially for skin contact or implantation, are almost exclusively latex-free. Common elastomeric components in tapes or wraps now use synthetic alternatives like polyurethane or silicone.
Q8: What is a “barbed” suture, and what material is it made from?
**A: A barbed suture is a monofilament (usually made from *polydioxanone* or polypropylene) with tiny, laser-cut barbs along its length. The barbs anchor the suture in tissue, distributing tension and often eliminating the need for knots. This is particularly useful in cosmetic and plastic surgery for even tissue approximation.**
Q9: How long does it take to develop and approve a new medical yarn material?
**A: The timeline is extensive, typically **5-10 years or more. It involves material synthesis and characterization, *in-vitro* testing, pre-clinical animal studies, clinical trials (for significant new materials), and a lengthy regulatory review process. This underscores the importance of working with established, compliant materials and suppliers.
Q10: As a textile manufacturer, what’s the first step to entering the medical yarn market?
**A: The first and most critical step is implementing a *compliant Quality Management System (QMS)*, specifically **ISO 13485. Without this foundational system governing design, production, and supplier management, it is impossible to be a credible supplier in the regulated medical device industry. Partnering with an experienced consultant in medical device regulation is highly recommended.










